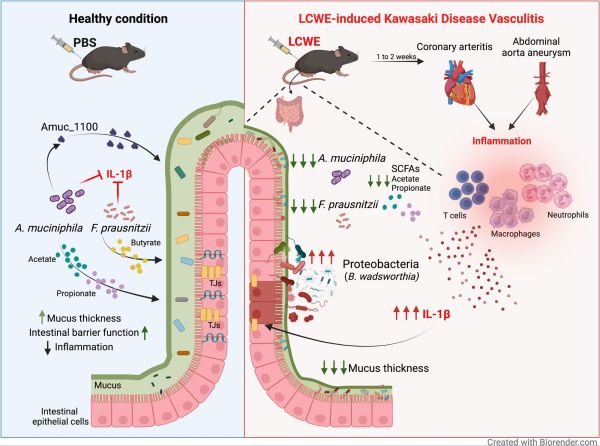
Alterations in the intestinal microbiota contribute to the pathogenesis of various cardiovascular disorders, but how they affect the development of Kawasaki disease (KD) an acute pediatric vasculitis, remains unclear.
METHODS:
We used the Lactobacillus casei cell wall extract (LCWE) murine model of KD vasculitis to assess the contribution of the intestinal microbiota to the development of vascular inflammation. We evaluated the severity of vasculitis in microbiota-depleted mice. 16S rRNA gene sequencing was used to characterize the fecal microbiome composition of LCWE-injected mice. Some groups of mice were orally treated with selected live or pasteurized bacteria, short-chain fatty acids, or Amuc_1100, the Toll-like receptor 2 signaling outer membrane protein from Akkermansia muciniphila, and their impact on vasculitis development was assessed.
RESULTS:
We report that depleting the gut microbiota reduces the development of cardiovascular inflammation in a murine model mimicking KD vasculitis. The development of cardiovascular lesions was associated with alterations in the intestinal microbiota composition and, notably, a decreased abundance of Akkermansia muciniphila and Faecalibacterium prausnitzii. Oral supplementation with either of these live or pasteurized individual bacteria or with short-chain fatty acids produced by them attenuated cardiovascular inflammation, as reflected by decreased local immune cell infiltrations. Treatment with Amuc_1100 also reduced the severity of vascular inflammation.
CONCLUSIONS:
This study reveals an underappreciated gut microbiota-cardiovascular inflammation axis in KD vasculitis pathogenesis and identifies specific intestinal commensals that regulate vasculitis in mice by producing metabolites or via extracellular proteins capable of enhancing and supporting gut barrier function.
https://www.ahajournals.org/doi/10.1161/CIRCRESAHA.124.325079